Our Work
Human Neurons and Synapses: Development, Function, and Disease
The research interests of our lab are to understand human genetic neuropathological conditions (such as autism, schizophrenia, epilepsy, and neurodegenerative diseases) as well as the molecular basis of human cellular neurobiology. We address questions such as: How does the brain develop? How does it work when a person thinks or feels? How do genes and proteins constitute mechanisms that lead to behavior?
The wiring of the nervous system during embryonic and early postnatal development is a prerequisite for the proper functioning of the human brain and is often impaired in neuropsychiatric diseases. Human pluripotent stem cell-derived neurons are a critical tool in this regard. Because of their translational relevance they are an excellent tool to understand the function of neuronal genes and proteins that impact neuronal development and synaptic functions.
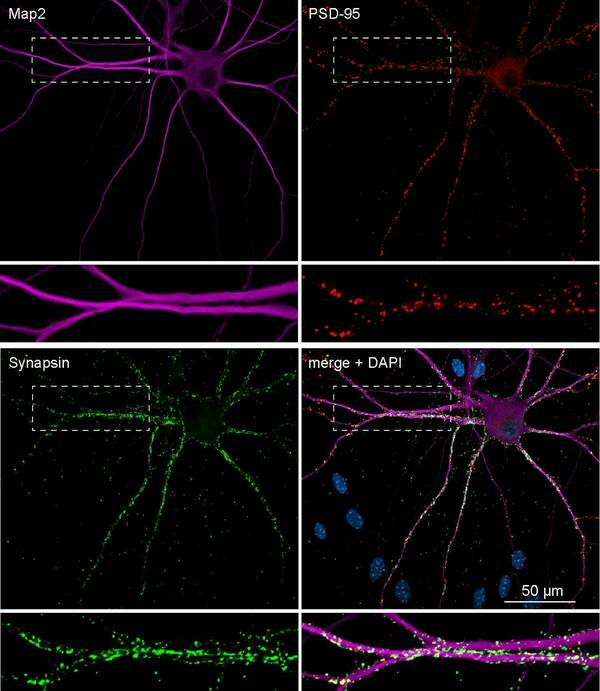
Robust synapse-formation as visible in this example of Ngn2-derived human neuron showing MAP2, postynaptic PSD95, and presynaptic Synapsin
The Conditional Knock-Out Approach: Cre/Lox Technology in Human Neurons
The use of human pluripotent stem cells (PSCs) to model human diseases has become a new standard in biomedical sciences. Particularly human neurons are impaired in their function in many congenital disorders. To this end, patient-derived somatic cells are studied in vitro to mimic human pathological conditions complementing and at least partly substituting animal models. As an alternative and supporting experimental strategy, we use the ‘conditional mutation approach’ which allows to engineer disease-relevant mutations in PSCs from healthy donors. In combination with a Cre/Lox technology approach, this strategy enables us to screen candidate mutations and the molecular causes of human diseases independent of the genetic background or genetic alterations induced by clonal selection.
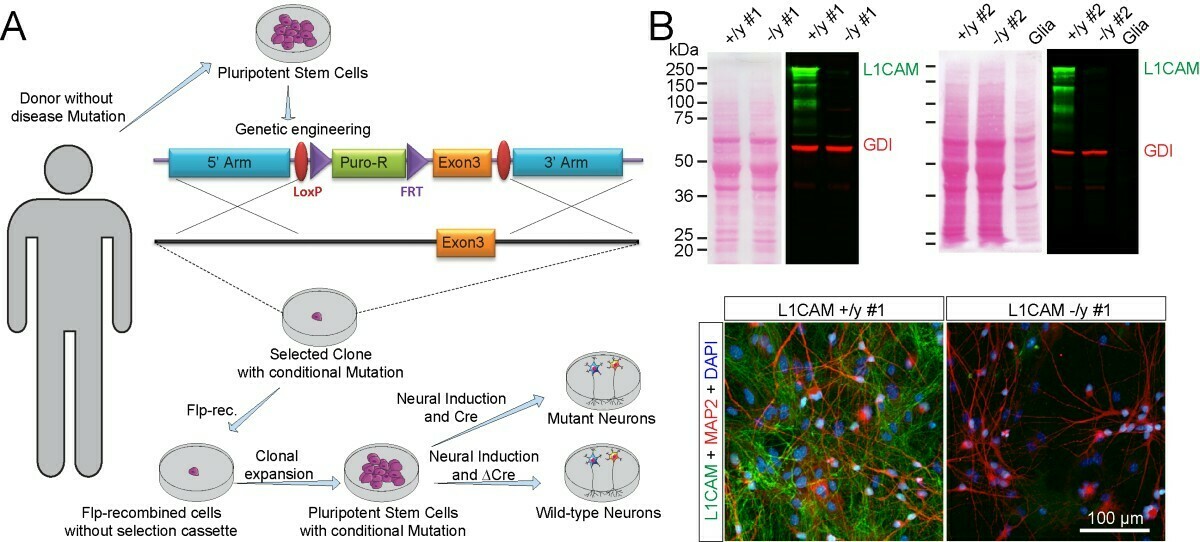
A Targeting strategy. The L1CAM gene was ‘floxed’ by homologous recombination in PSCs. B Ponceau-stained blotting membranes (left) and immunoblots (right) of human neurons derived from two independent L1CAM clones (#1 and #2). Immunoblots were stained with antibodies to human L1CAM (green) and GDI (red; loading control); lower panel: Representative immunofluorescently labeled control and mutant L1CAM-deficient neurons. L1CAM, MAP2, and DAPI staining is shown. Modified from Patzke et al. 2016, JEM.
Neuromodulator Signaling Bidirectionally Controls Vesicle Numbers in Human Synapses
The phosphoprotein family of synapsins in human neurons have the ability to acutely and bi-directionally regulate the number of presynaptic vesicles in the reserve pool of nerve terminals. Patient-mutations in the X-chromosomally located Synapsin-1 gene are also highly linked to epilepsy and mental retardation. Our results not only reveal the ultra-structure of the human synapse in vitro (by fast-freeze EM, in collaboration with the lab of Dr. Christian Rosenmund, Charité Berlin) but also show that after stimulation by neuromodulators synapsins acutely control in a cAMP-dependent manner the reserve pool size of synaptic vesicles in human neurons by acting downstream of neuromodulator G-protein coupled receptors (GPCR) for serotonin and noradrenaline. These findings describe a completely novel and unexplored pathway for presynaptic plasticity regulation during synaptic transmission, which is linked to protein kinase A phosphorylating the N-terminus of synapsins.
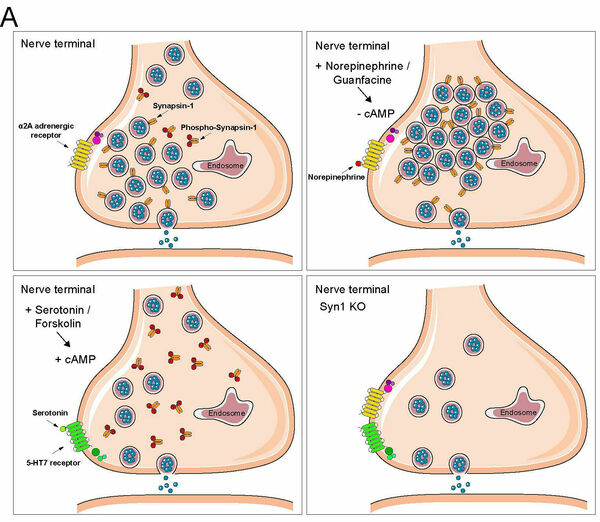
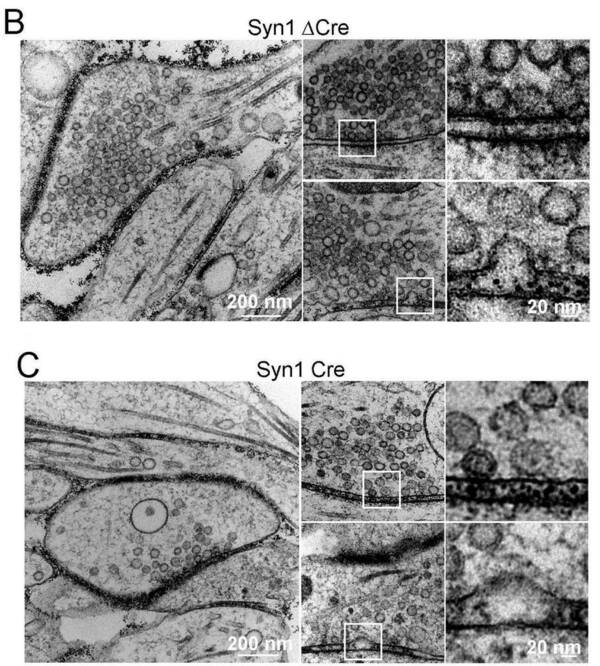
A Neuromodulator-GPCR signaling rapidly changes synaptic vesicle numbers by changing cAMP-dependent Synapsin-1 phosphorylation. B and C Electron micrographs showing reduced number of synaptic vesicles in the reserve pool of human neurons after deletion of Synapsin-1 and optogenetic stimulation. Modified from Patzke, Brockmann, Dai et al. 2019, Cell.
Cannabinoid Receptor Activation Acutely Increases Synaptic Vesicle Numbers by Activating Synapsins in Human Synapses
Cannabinoids as medical agents are in the focus of the scientific and clinical community as well known neuromodulators. Their action in the brain is mainly characterized as synaptic messengers activating presynaptic CB1 receptors, which then suppress synaptic transmission by inhibiting Ca2+ channels. We show that acute CB1 receptor activation can rapidly modulate synaptic vesicle numbers and neurotransmitter release in human synapses by ‘activating’ synapsin, which is a novel way to regulate transmission - a node of regulation that has not been considered previously and bypasses inhibition of Ca 2+ channels. Extending these findings to G-protein coupled endocannabinoid signaling, we can show that independentaly of Ca 2+ channels, synapsins acutely control presynaptic vesicle numbers in mouse and human nerve terminals upon CB1-receptor activation.
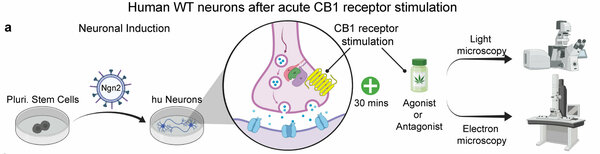
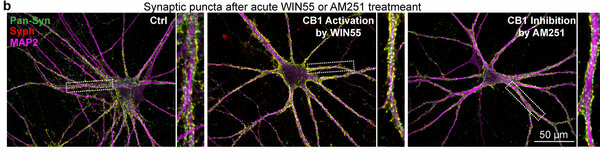
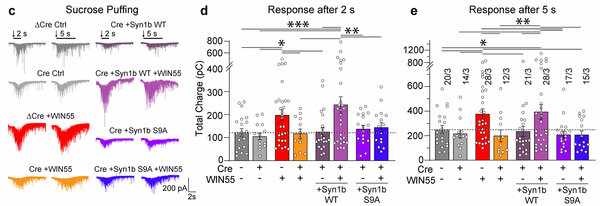
A By lentiviral transduction of the transcription factor Ngn2, pluripotent stem cells are converted into mature human neurons, which are acutely treated with ‘agonists’ (WIN55, ACEA, AEA or 2-AG) or ‘antagonists’ (inverse agonist AM251 or competitive antagonist CP945598) for CB1 receptors. Neurons are scored for morphologic and ultra-structural synaptic changes. B Human neurons before and after acute treatment (30 mins) for activation of CB1 receptors with WIN55 or inhibition with AM251. C-E Traces and measurements of neurotransmitter release induced by puffing hypertonic sucrose for duration of 2 s and 5 s to assess the size of the readily releasable pool (RRP) of vesicles. Modified from Patzke, Dai, Brockmann et al. 2021, Molecular Psychiatry.
Neurodegenerative Diseases
PSC-derived human neurons have the ability to live for up to two years in culture. This makes them a very versatile tool to study neuronal aging and degenerative processes in health and disease.
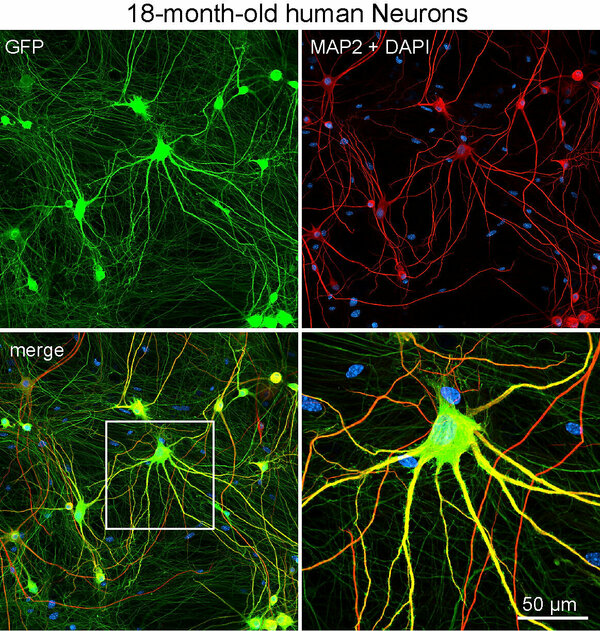
Human neurons transfected with GFP and stained for MAP2 and DAPI after 18 months in culture.